Product Description
SUMMARY
Urine undergoes many changes during states of disease or body dysfunction before blood composition is altered to a significant extent. Urinalysis is a useful procedure as an indicator of health or diseases, and as such, is a part of routine health screening. Urine Reagent Strips can be used in general evaluation of health, and aids in diagnosis and monitoring or metabolic or systemic diseases that affect the kidney function, endocrine disorders and diseases or disorders of the urinary tract.
INTENDED USE
Urine Reagent Strips are firm plastic strips onto which several separate reagent areas are affixed. The test is for detection of one or more of the following analytes in urine: Leukocytes, Glucose, Ketone (Acetoacetic acid), Bilirubin, Blood, Specific Gravity, Protein, Urobilinogen, Nitrite and pH.
PRECAUTIONS
- For in-vitro diagnostic use only. Do not use after the expiration date.
- The strip should remain in the sealed pouch until use.
- Do not touch the reagent areas of the strip.
- Discard any discoloured strips that may have deteriorated.
- All specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
- The used strip should be discarded according to local regulations after testing.
STORAGE & STABILITY
Store as packaged in the sealed pouch at room temperature between 15-30°C. Keep out of direct sunlight. The strip is stable through the expiration date printed on the outer box and pouch. DO NOT FREEZE. Do not use beyond the expiration date.
How To Use
SPECIMEN COLLECTION & PREPARATION
A urine specimen must be collected in a clean and dry container and tested as soon as possible. Do not centrifuge. The use of urine preservatives is not recommended. If testing cannot be done within an hour after voiding, refrigerate the specimen immediately and let it return to room temperature before testing. Prolonged storage of unpreserved urine at room temperature may result in microbial proliferation with resultant changes in pH. A shift to alkaline pH may cause false positive results with the protein test area. Urine containing glucose may decrease in pH as organisms metabolize the glucose.
Contamination of the urine specimen with skin cleansers containing chlorhexidine may affect protein (and to a lesser extent, specific gravity and bilirubin) test results.
MATERIALS
Materials Provided: Test Strips and Instruction for Use (IFU)
Materials Required but Not Provided: Timer.
DIRECTION FOR USE
Remove the strip from the sealed pouch and use it as soon as possible. Completely immerse the reagent areas of the strip in fresh, well-mixed urine and immediately remove the strip to avoid dissolving the reagents.
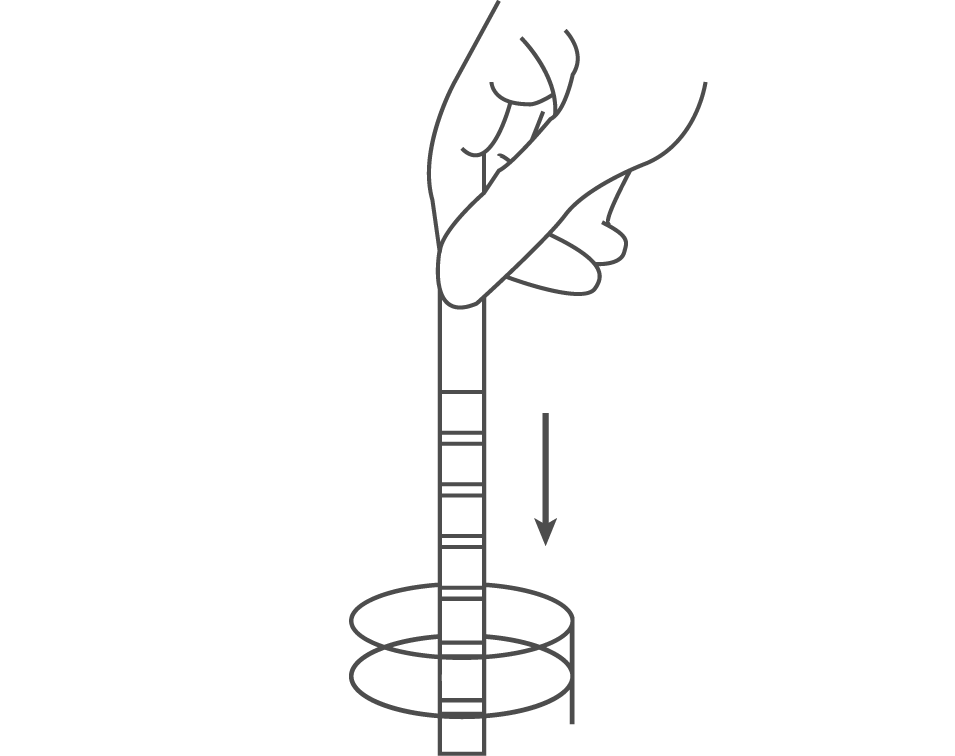
While removing the strip from the urine, run the edge of the strip against the rim of the urine container to remove excess urine. Hold the strip in a horizontal position and bring the edge of the strip into contact with an absorbent material (e.g. a paper towel) to avoid mixing chemicals from adjacent reagent areas and/or soiling hands with urine.
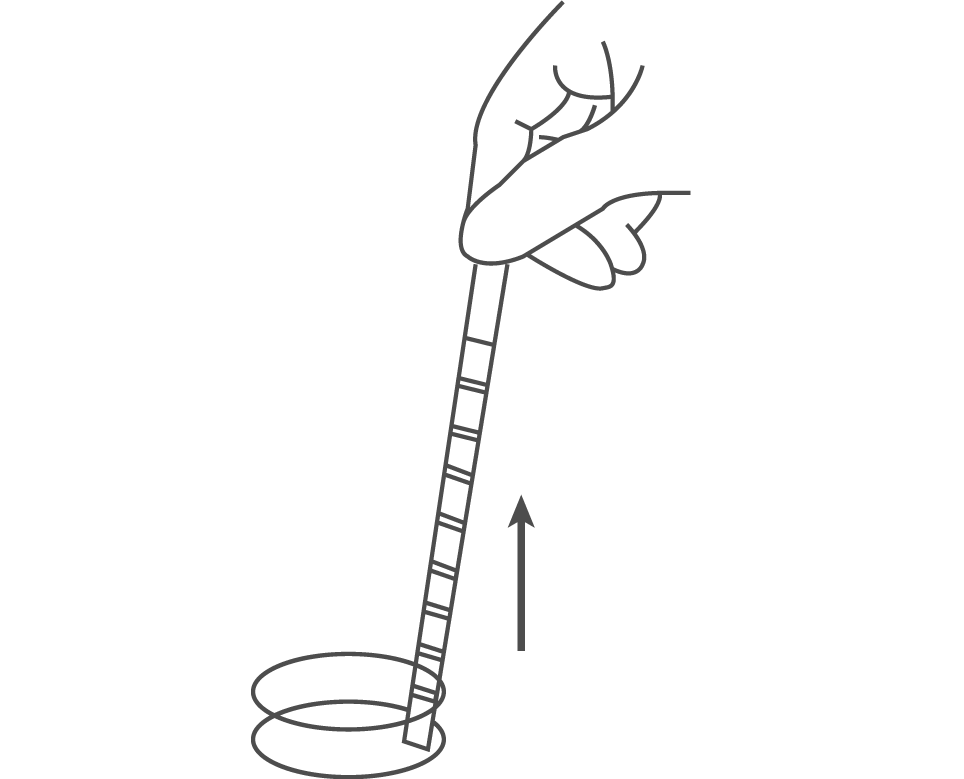
Compare the reagent areas to the corresponding colour blocks on the outer box label at the specified times. Hold the strip close to the colour blocks and match carefully. Results may be read up to 2 minutes after the specified times.
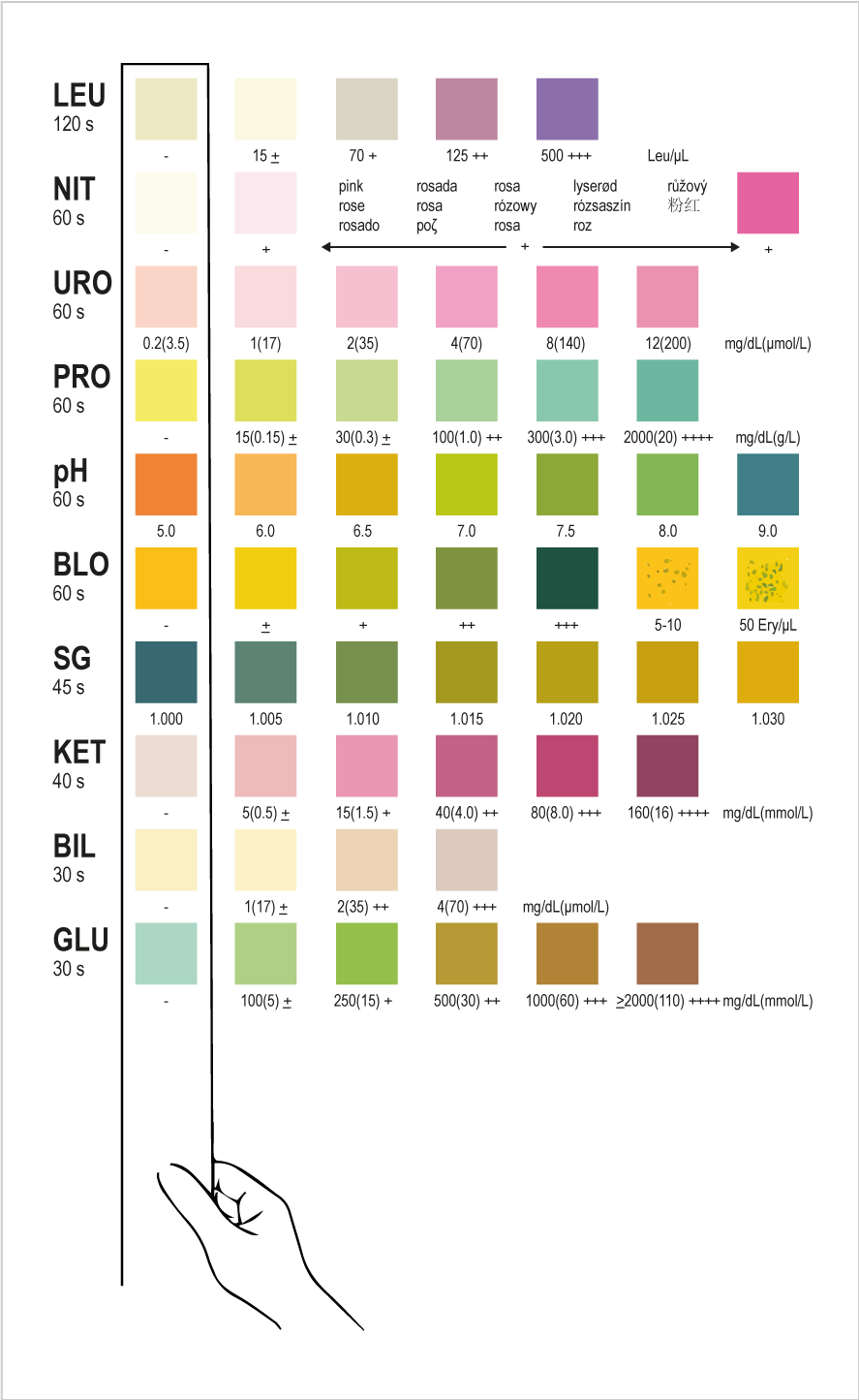
INTERPRETATION OF RESULTS
Results are obtained by direct comparison of the colour blocks printed on the outer box label. The colour blocks represent nominal values; actual values will vary close to the nominal values. In the event of unexpected or questionable results, the following steps are recommended; confirm that the specimens have been tested within the expiration date printed on the outer box and pouch, compare results with known positive and negative controls and repeat the test using a new strip. If the problem persists, discontinue using the strip immediately and contact your local distributor.
More About Urine Reagent Strips
PRINCIPLE AND EXPECTED VALUES
Leukocytes: This test reveals the presence of granulocyte esterases. The esterases cleave a derivatized pyrazole amino acid ester to liberate derivatized hydroxy pyrazole. This pyrazole then reacts with a diazonium salt to produce a beige-pink to purple colour. Normal urine specimens generally yield negative results. Trace results may be of questionable clinical significance. When trace results occur, it is recommended to retest using a fresh specimen from the same patient. Repeated trace and positive results are of clinical significance.
Glucose: This test is based on the enzymatic reaction that occurs between glucose oxidase, peroxidase and chromogen. Glucose is the first oxidized to produce gluconic acid and hydrogen peroxide in the presence of glucose oxidase. The hydrogen peroxide reacts with potassium iodide chromogen in the presence of peroxidase. The extent to which the chromogen is oxidized determines the colour, which is produced, ranging from green to brown. Low amounts of glucose are normally excreted in urine. Glucose concentrations as low as 100 mg/dL, read at either 10 or 30 seconds, may be considered abnormal if results are consistent. At 10 seconds, results should be interpreted qualitatively. For semi-quantitative results, read at 30 seconds only.
Ketone: This test is based on ketones reacting with nitroprusside and acetoacetic acid to produce a colour change ranging from light pink for negative results to a darker pink or purple colour for positive results. Ketones are normally not present in urine. Detectable ketone levels may occur in urine during physiological stress conditions such as fasting, pregnancy and frequent strenuous exercise. In starvation diets, or in other abnormal carbohydrate metabolism situations, ketones appear in the urine in excessively high concentration before seum ketones are elevated.
Bilirubin: This test is based on azo-coupling reaction of bilirubin with diazotized dichloroaniline in a strongly acidic medium. Varying bilirubin levels will produce a pinkish tan colour proportional to its concentration in urine. In normal urine, no bilirubin is detectable by even the most sensitive methods. Even trace amounts of bilirubin require further investigation. Atypical results (colours different from the negative or positive colour blocks shown on the colour chart) may indicate that bilirubin-derived bile pigments are present in the urine specimen and are possibly masking the bilirubin reaction.
Blood: This test is based on the peroxidase-like activity of hemoglobin which catalyzes the reaction of cumene-hydroperoxide and 3, 3’, 5, 5’-tetramethylbenzidine. The resulting colour ranges from orange to green to dark blue. Any green spots or green colour development on the reagent area within 60 seconds is significant and the urine specimen should be examined further. Blood is often, but not invariably, found in the urine of menstruating females.
Specific Gravity: This test is based on the apparent pKa change of certain pre-treated polyelectrolytes in relation to ionic concentration. In the presence of an indicator, colours range from deep blue-green in urine of low ionic concentration to green and yellow-green in urine of increasing ionic concentration. Randomly collected urine may vary in specific gravity from 1.003-1.040. Twenty-four-hour urine from healthy adults with normal diets and fluid intake will have a specific gravity of 1.016-1.022. In cases of severe renal damage; the specific gravity is fixed at 1.010, the value of the glomerular filtrate.
Protein: This reaction is based on the phenomenon known as the “protein error”, of pH indicators where an indicator that is highly buffered will change colour in the presence of proteins (anions) as the indicator releases hydrogen ions to the protein. At a constant pH, the development of any green colour is due to the presence of protein. Colours range from yellow to yellow-green for negative results and green to green-blue for positive results. 1-14 mg/dL of protein may be excreted by a normal kidney. A colour matching any block greater than trace indicates significant proteinuria. For urine with high specific gravity, the test area may most closely match the trace colour block even though only normal concentrations of protein are present. Clinical judgment is required to evaluate the significance of trace results.
Urobilinogen: This test is based on a modified Ehrlich reaction between p-diethylaminobenzaldehyde and urobilinogen acidin strongly acidic medium to produce a pink colour. Urobilinogen is one of the major compounds produced in heme synthesis and is a normal substance in urine. The expected range for normal urine with this test is 0.2-1.0 mg/dL (3.5-17 mmol/L). A result of 2.0 mg/dL (35 mmol/L) may be of clinical significance, and the patient specimen should be further evaluated.
Nitrite: This test depends upon the conversion of nitrate to nitrite by the action of Gram-negative bacteria in the urine. In an acidic medium, nitrite in the urine reacts with p-arsanilic acid to form a diazonium compound. The diazonium compound in turn couples with 1N-(1-naphthyl)-ethylenediamineto to produce a pink colour. Nitrite is not detectable in normal urine. The nitrite area will be positive in some cases of infection, depending on how long the urine specimens were retained in the bladder pval of positive cases with the nitrite test range from as low as 40% in cases where little bladder incubation occurred, to as high as approximately 80% in cases where bladder incubation took place for at least 4 hours.
pH: This test is based on a double indicator system which gives a broad range of colours covering the entire urinary pH range. Colours range from orange to yellow and green to blue. The expected range for normal urine specimens from new-borns is pH 5-7. The expected range for other normal urine specimens is pH 4.5-8, with an average result of pH 6.
REAGENTS AND PERFORMANCE CHARACTERISTICS
ex.
Reagant / Read Time
Composition
Description
- 1.5% w/w glucose oxidase; 0.5% w/w peroxidase; 2.0% w/w o-tolidine; 75.0% 1/1 buffer; 2.0% w/w non-reactive ingredients
- Detects glucose as low as 25-50 mg/dL (1.4~2.8 mmol/L). Results may be read at 20 seconds for qualitative results or at 45 seconds for semi-quantitative results.
- 5% w/w sodium nitroprusside; 95% w/w buffer
- Detects acetoacetic acid as low as 2.5-5 mg/dL (0.25-0.5 mmol/L).
- 0.5% w/w 2, 4-dichloroaniline diazonium salt; 99.5% w/w buffer and non-reactive ingredients
- Detects bilirubin as low as 0.4-0.8 mg/dL (6.8-13.6 μmol/L)
- 4% w/w 3,3’, 5,5’-tetramethylbenzidine (TMB); 6% w/w cumene hydroperoxide; 90% w/w buffer and non-reactive ingredients
- Detects free hemoglobin as low as 0.015-0.062 mg/dL or 5-10 Ery/μL in urine specimens with ascorbic acid content of < 50 mg/dL.
- 2.5% w/w bromthymol blue indicator; 17.5% w/w buffer and non-reactive ingredients; 55% poly (methyl vinyl ether/maleic anhydride); 25% sodium hydroxide
- Determines urine specific gravity between 1.000 and 1.030. Results correlate with values obtained by refractive index method within ±0.005.
- 0.3% w/w tetrabromophenol blue; 99.7% w/w buffer and non-reactive ingredients
- Detects albumin as low as 7.5-20 mg/dL (0.075-0.2g/L).
- 2.5% w/w p-diethylaminobenzaldehyde; 97.5% w/w buffer and non-reactive ingredients
- Detects urobilinogen as low as 0.2-1.0 mg/dL (2.5-17μmol/L).
- 4.5% w/w p-arsanilic acid; 95.5% w/w non-reactive ingredients
- Detects sodium nitrite as low as 0.05-0.1 mg/dL in urine with a low specific gravity and less than 30 mg/dL ascorbic acid.
- 0.5% w/w methyl red sodium salt; 5% w/w bromthymol blue; 94.5% w/w non-reactive ingredients
- Permits the quantitative differentiation of pH values within the range of 5-9.
- 0.5% w/w derivatized pyrrole amino acid ester; 0.4% w/w diazonium salt; 32% w/w buffer; 67.1% w/w non-reactive ingredients
- Detects leukocytes as low as 10-25 white blood cells Leu/mL in clinical urine.
The performance characteristics of the Urine Reagent Strips have been determined in both laboratory and clinical tests. Parameters of importance to the users are sensitivity, specificity, accuracy and precision. Generally, this test has been developed to be specific for the parameters to be measured with the exceptions of the interferences listed. Please refer to the Limitations section in this Instruction for Use (IFU).
Interpretation of visual results is dependent on several factors: the variability of colour perception, the presence or absence of inhibitory factors, and the lighting conditions when the strip is read. Each colour block on the chart corresponds to a range of analyte concentrations.
QUALITY CONTROL
For best results, performance of reagent strips should be confirmed by testing known positive and negative specimens/controls whenever a new test is performed. Each laboratory should establish its own goals for adequate standards of performance.
LIMITATIONS
Note: as with all diagnostic and therapeutic tests, all results must be considered with other clinical information available to the physician.
Glucose: This test is highly specific for glucose. No substance excreted in urine other than glucose is known to give a positive result. The reagent area does not react with ketones, lactose, galactose, fructose or other metabolic substances, nor with reducing metabolites of drugs (e.g. salicylates and nalidixic acid). Sensitivity may be decreased in specimens with high specific gravity (>1.025) and with ascorbic acid concentrations of ≥ 10 mg/dL.
Ketone: The test does not react with acetone or β-hydroxybutyrate. Urine specimens of high pigment, and other substances containing sulfhydryl groups occasionally give reactions up to and including trace (+).
Bilirubin: Bilirubin is absent in normal urine, so any positive result, including a trace positive, indicates an underlying pathological condition and requires further investigation. Reactions may occur with urine containing large doses of chlorpromazine orrifampen that might be mistaken for positive bilirubin. The presence of bilirubin-derived bile pigments may mask the bilirubin reaction. This phenomenon is characterized by colour development on the test patch that does not correlate with the colours on the colour chart. Large concentrations of ascorbic acid may decrease sensitivity.
Blood: A uniform blue colour indicates the presence of myoglobin, hemoglobin or hemolyzed erythrocytes. Scattered or compacted blue spots indicate intact erythrocytes. To enhance accuracy, separate colour scales are provided for hemoglobin and for erythrocytes. Positive results with this test are often seen with urine from menstruating females. It has been reported that urine of high pH reduces sensitivity, while moderate to high concentration of ascorbic acid may inhibit colour formation. Microbial peroxidase, associated with urinary tract infection, may cause a false positive reaction. The test is slightly more sensitive to free hemoglobin and myoglobin than to intact erythrocytes.
Specific Gravity: Ketoacidosis or protein higher more than 100mg/dL may cause elevated results. Results are not affected by non-ionic urine components such as glucose. If the urine has a pH of 7 or greater, add 0.005 to the specific gravity reading indicated on the colour chart.
Protein: Any green colour indicates the presence of protein in the urine. This test is highly sensitive for albumin, and less sensitive to hemoglobin, globulin and mucoprotein. A negative result does not rule out the presence of these other proteins. False positive results may be obtained with highly buffered or alkaline urine. Contamination of urine specimens with quaternary ammonium compounds or skin cleansers containing chlorhexidine produces false positive results. The urine specimens with high specific gravity may give false negative results.
Urobilinogen: All results lower than 1mg/dL urobilinogen should be interpreted as normal. A negative result does not at any time preclude the absence of urobilinogen. The reagent area may react with interfering substances known to react with Ehrlich’s reagent, such as p-aminosalicylic acid and sulfonamides. False negative results may be obtained if formalin is present. The test cannot be used to detect porphobilinogen.
Nitrite: The test is specific for nitrite and will not react with any other substance normally excreted in urine. Any degree of uniform pink to red colour should be interpreted as a positive result, suggesting the presence of nitrite. Colour intensity is not proportional to the number of bacteria present in the urine specimen. Pink spots or pink edges should not be interpreted as a positive result. Comparing the reacted reagent area on a white background may aid in the detection of low nitrite levels, which might otherwise be missed. Ascorbic acid above 30 mg/dL may cause false negatives in urine containing less than 0.05 mg/dL nitrite ions. The sensitivity of this test is reduced for urine specimens with highly buffered alkaline urine. For accurate results, antibiotics should be discontinued for at least 3 days before the test if performed. A negative result does not at any time preclude the possibility of bacteruria. Negative results may occur in urinary tract infections from organisms that do not contain reductase to convert nitrate to nitrite; when urine has not been retained in the bladder for a sufficient length of time (at least 4 hours) for reduction of nitrate to nitrite to occur; or when dietary nitrate is absent.
pH: pH readings are not affected by variations in urinary buffer concentration.
Leukocytes: The result should be read between 60-120 seconds to allow for complete colour development . The intensity of the colour that develops is proportional to the number of leukocytes present in the urine specimen. High specific gravity or elevated glucose concentrations (≥ 500 mg/dL) may cause test results to be artificially low. The presence of cephalexin, cephalothin, or high concentrations of oxalic acid may also cause test results to beartifically low. Tetracycline may cause decreased reactivity, and high levels of the drug may cause a false negative reaction. High urinary protein (≥ 500 mg/dL) may diminish the intensity of the reaction colour. This test will not react with erythrocytesor bacteria common in urine.
BIBLIOGRAPHY
- Free AH, Free HM. Urinalysis, Critical Disciplne of Clinical Science. CRC Crit. Rev. Clin. Lab. Sci. 3(4): 481-531, 1972.
- Yoder J, Adams EC, Free, AH. Simultaneous Screening for Urinary Occult Blood, Protein, Glucose, and pH. Amer. J. Med Tech. 31:285, 1965.
- Shchersten B, Fritz H. Subnormal Levels of Glucose in Urine. JAMA 201:129-132, 1967.
- McGarry JD, Lilly. Lecture, 1978: New Perspectives in the Regulation of Ketogenesis. Diabetes 28: 517-523 May, 1978.
- Williamson DH. Physiological Ketoses, or Why Ketone Bodies? Postgrad. Med. J. (June Suppl.): 3720375, 1971.
- Paterson P, et al. Maternal and Fetal ketone Concentrations in Plasma and Urine. Lancet: 862-865; April 22, 1967.
- Fraser J, et al. Studies with a Simplified Nitroprusside Test for Ketone Bodies in Urine, Serum, Plasma and Milk. Clin. Chem. Acta II: 372-378, 1965.
- Henry JB, et al. Clinical Diagnosis and Management by Laboratory Methods, 18th Ed. Philadelphia. Saunders. 396-397, 415, 1991.
- Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemistry 2ndEd. 2205, 1994.
- Tietz NW. Clinical Guide to Laboratory Test. W.B. Saunders Company. 1976.
BTI1452
IFU-BT-TK035-m.v2.2019
Manufactured by Atlas Medical
Ludwig-Erhard-Ring 3, 15827 Blankenfelde-Mahlow, Berlin, Germany



